A General Strategy to Access Hyperstable and Ultrashort Collagen Heterotrimers
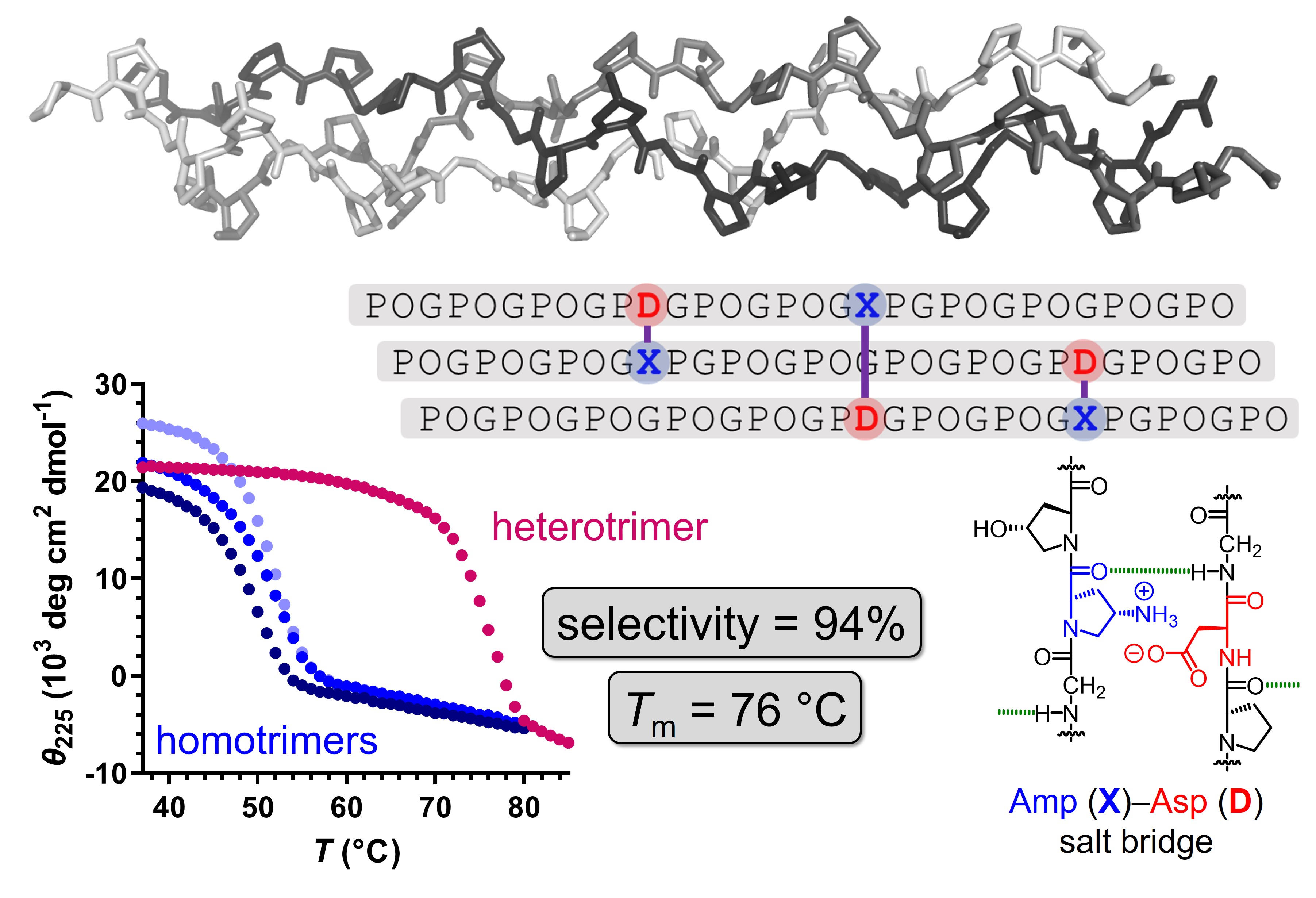
Most types of natural collagen are heterotrimers comprised of three strands that assemble into a triple helix. Despite the significance of collagen as a biomaterial – one third of all proteins in mammals is collagen – the isolation of pure collagen is a challenge due to the large size, crosslinking and numerous heterogenous post-translational modifications. Collagen model peptides (CMPs) have emerged as synthetic surrogates for investigating collagen structure and developing biological materials and probes. However, the selective assembly of CMPs into heterotrimers is challenging since a combination of three different CMPs can form up to 27 distinct triple helices. In this study, we demonstrate that salt bridges between (4S)-aminoproline (Amp) and aspartate (Asp) can be employed as a robust and universal driving force for the selective assembly of collagen heterotrimers. By introducing a single Amp-Asp salt bridge between each pair of strands, we assembled the shortest stable supramolecular collagen heterotrimer (with 17-mer strands) as well as the most stable selectively assembled collagen heterotrimer (32-mer strands, melting temperature 76 °C) reported to date. Our work provides a modular toolbox for the development of heterotrimeric collagen-based materials and probes.