A Locally Activatable Sensor for Robust Quantification of Organellar GSH
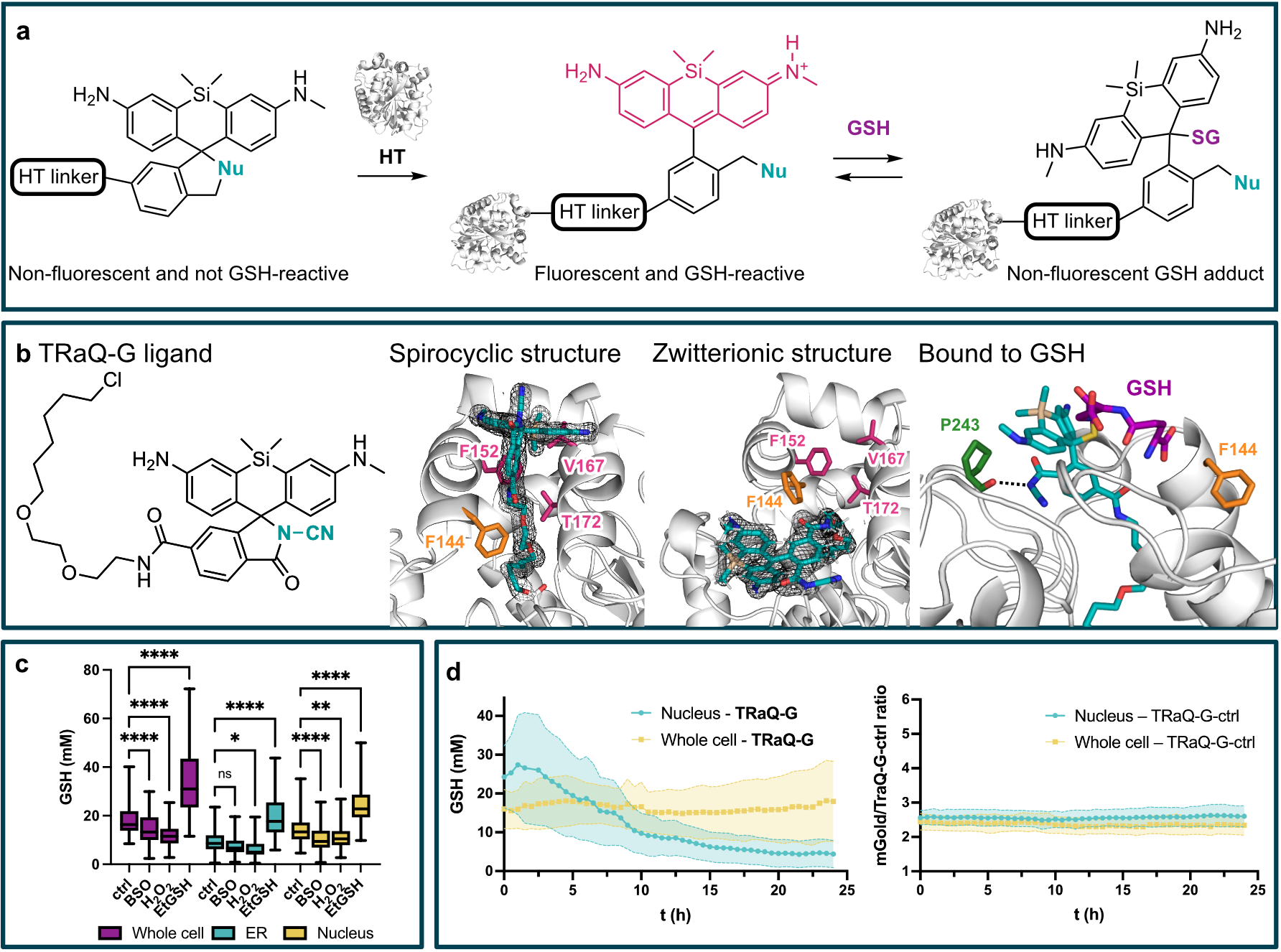
Glutathione (GSH) is the main determinant of intracellular redox potential and participates in multiple cellular signaling pathways. Achieving a detailed understanding of intracellular GSH trafficking and regulation depends on the development of tools to map GSH compartmentalization and intra-organelle fluctuations. Here, we propose a Targetable Ratiometric Quantitative GSH sensor (TRaQ-G)[1] for live-cell imaging based on the previously reported reactivity of certain silicon rhodamines towards GSH.[2] A nucleophilic group on our reporter fluorophore leads in the unbound state to a spirocyclic, non-fluorescent conformation. Only upon binding to the HaloTag (HT) protein, the fluorescence and the GSH-responsiveness is switched on (panel a). Fusing HT to a fluorescent protein and a targeting peptide, allows for a robust ratiometric read-out and flexible targeting. Our structural analysis including X-ray crystal structures and molecular dynamics simulations (panel b) allowed us to reason the sensitivity of our sensor molecule towards GSH. We confirmed that our sensor reliably responds to artificially induced changes in GSH concentration in several subcellular compartments (panel c). Furthermore, we studied GSH regulation during the cell cycle by imaging synchronized HeLa cells with our sensor (panel d). Our results indicate a separately regulated nuclear GSH pool with an especially high concentration right before cell division which matches previous reports.[3]
[2] K. Umezawa, M. Yoshida, M. Kamiya, T. Yamasoba, Y. Urano, Nat. Chem. 2017, 9, 279–286.
[3] J. L. García-Giménez, J. Markovic, F. Dasí, G. Queval, D. Schnaubelt, C. H. Foyer, F. V. Pallardó, Biochim. Biophys. Acta 2013, 1830, 3304–3316.