A General Strategy to Develop Fluorogenic Polymethine Dyes for Bioimaging
Fluorescence imaging is an invaluable tool to study biological processes and further progress in this research area depends on the development of advanced probes. Fluorogenic dyes are crucial to reach intracellular targets and label them with high specificity. Excellent fluorogenic rhodamine dyes have been reported, but they often require a long and low-yielding synthesis and are spectrally limited to the visible range.
Here, we present a general strategy to transform polymethine compounds into fluorogenic dyes using a 5-exo-trig ring closure approach1, in contrast to previously attempted 5-endo-trig ring-closures2 (Panel a-b). These dyes, regardless of their excitation wavelength, can be readily synthesized in two steps and are easy to derivatize by varying the indoleninium building blocks or the ring-closing moiety. We demonstrate that a 5-exo-trig Cy5 probe conjugated to the self-labeling protein tag SNAP-tag shows high fluorogenicity and a bright and specific fluorescence signal in live HeLa cells, whereas the corresponding 5-endo-trig probe showed low cell permeability, low fluorogenicity and high unspecific fluorescence signal (Panel c).
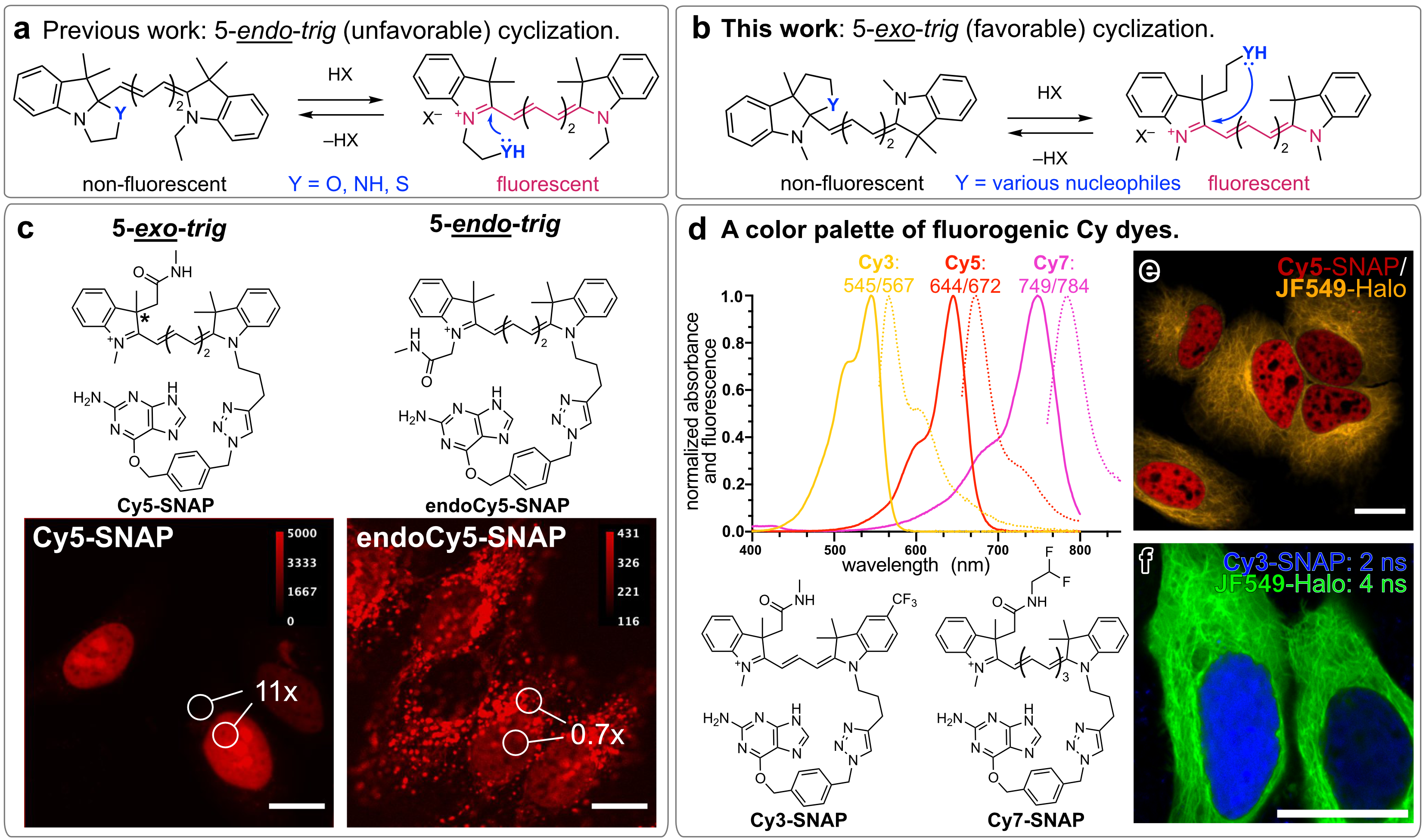
We illustrate the generality of this method by creating the first spontaneously blinking Cy5 dye as well as no-wash, turn-on polymethine dyes with emissions across the visible and near-infrared spectrum (Panel d). These probes are not only compatible with self-labeling proteins but also with small-molecule targeting ligands such as jasplakinolide and Hoechst 33342 dye and can be combined with rhodamine-based dyes for multicolor (Panel e) and fluorescence lifetime multiplexing imaging (Panel f). We envision that our simple, yet general, method will be useful to develop improved fluorogenic probes in the future, thus facilitating new bioimaging experiments.
[2] examples from the Ohe group include a) K. Miki et al., Chem. Commun. 2017, 53, 7792–7795. b) M. Oe et al.,
Tetrahedron Lett. 2018, 59, 3317–3321. c) M. Oe et al., Chem. Commun. 2022, 58, 1510–1513.