Semipinacol Rearrangement of Cyclopropenylcarbinols for the Synthesis of Highly Substituted Cyclopropanes
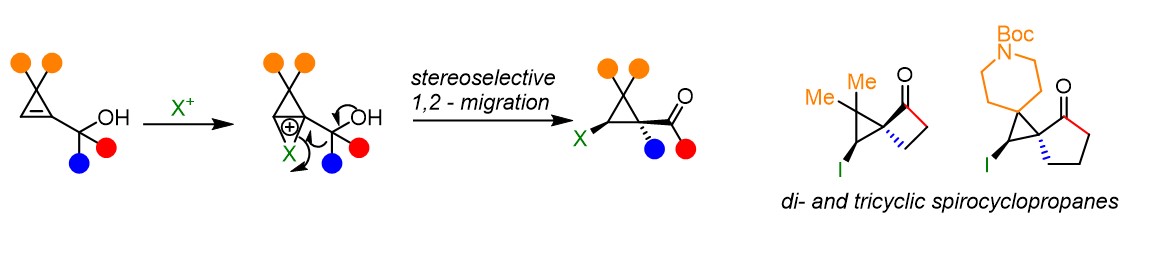
Since their discovery, cyclopropanes have attracted the attention of chemists due to their unique bonding properties, their ring strain, and their rigid conformation. In addition, cyclopropanes are present in numerous bioactive compounds, including both natural products and synthetic molecules. Nevertheless, polysubstituted cyclopropanes are difficult to access. One successful approach relies on the functionalization of cyclopropenes via carbo- or heterometallation of the double bond, which allows the simultaneous introduction of two substituents on the three-membered ring in a stereoselective fashion.[1] In contrast, the electrophilic activation of the cyclopropene double bond is less developed.[2] Herein, we report electrophile-induced semipinacol rearrangement of cyclopropenyl carbinols leading to the stereoselective synthesis of functionalized cyclopropyl ketone products. The transformation is efficient for the generation of di- and tri-spirocyclic compounds, containing cyclopropanes and cyclobutanes. Soft electrophiles, such as I+, PhS+, PhSe+, and NCS+, promoted efficiently the rearrangement. The 1,2-migration was achieved in the case of aryl-substituted alcohols, cyclopropanols, and cyclobutanols. The utility of the obtained products was demonstrated by variety of product modifications. Our work demonstrates the high synthetic potential of the electrophilic activation of cyclopropenes as an underdeveloped strategy for synthesizing complex cyclopropanes.
[2] a) V. R. Kartashov, E. V. Skorobogatova, N. S. Zefirov, Russ. Chem. Rev. 1993, 62, 935. b) H. Mizoguchi, M. Seriu, A. Sakakura, Chem. Commun. 2020, 56, 15545–15548.