Electrochemically Catalyzed Nitration of Unsaturated Hydrocarbons
Catalysis is a key feature in organic chemistry and one of the most important and interesting areas of discovery in academic and industry research. Chemical and enzymatic catalysis has been recognized a least 16 times by the Nobel Foundation. Among different catalysts such as transition metals, enzymes, organocatalysts, Lewis base and Brønstead acids, the proton is the smallest, whereas the electron is a tiny element. The field of electron as a catalyst was only recently conceptualized, and is yet lacking practical examples to attract attention of the scientific community. The electron as a catalyst is identified in catalysis as inexpensive, traceless, and green. Moreover, in addition to having the highest mass efficiency among others catalysts, it does not need to be removed from the reaction mixture. In recent years several electrochemical protocols have been reported in which transition metals and electrons in superstoichiometric amounts have been used to promote the catalytic cycle.1,2 However, in an ideal electrocatalytic reaction, a sub-stoichiometric amount of electrons is required to complete the process.
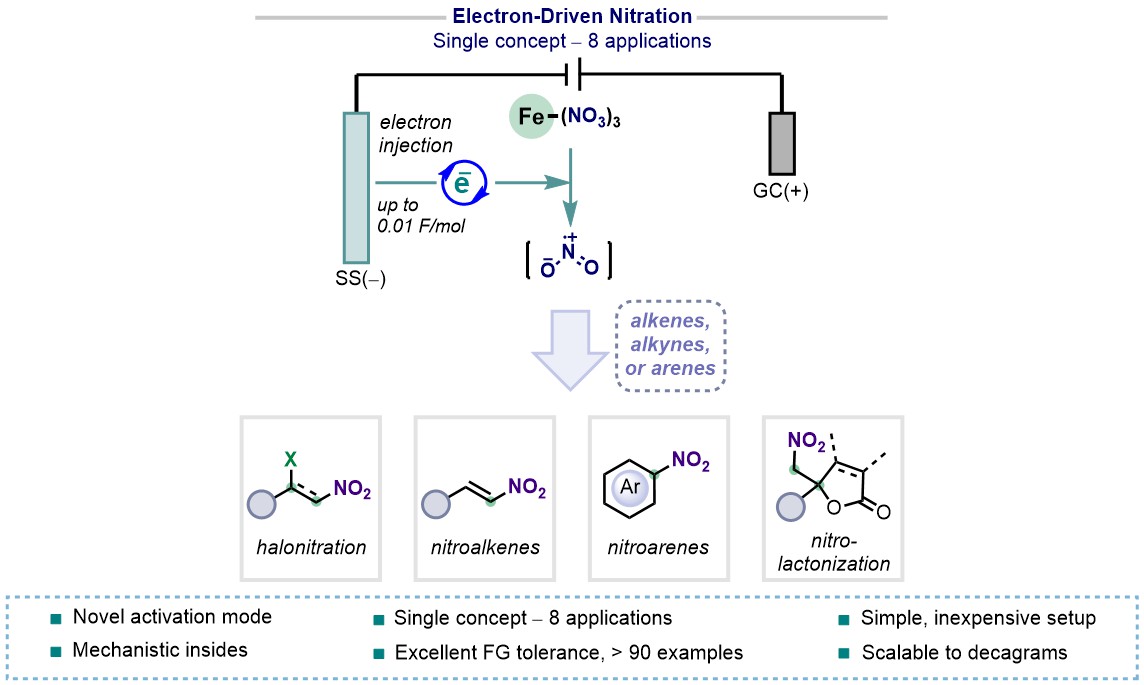
Herein, we introduce an electrochemically catalyzed paradigm for the generation of nitryl radicals from ferric nitrate under mild and additive-free reaction conditions using a simple setup with inexpensive graphite and stainless steel electrodes. Detailed mechanistic studies and controlled experiments of such a unique activation mode of iron nitrate was examined by combined spectroscopic and detailed experimental studies and revealed that the reaction proceeds via a radical pathway in the presence of nitryl radicals and operates under catalytic electrons. This operationally simple electron-mediated protocol offers straightforward access to a variety of nitro-derived molecules from unsaturated hydrocarbons including alkenes, alkynes, arenes, and also efficiently promotes a series of ipso-nitration reactions and nitrative cyclizations with high levels of chemo- and regioselectivity. In addition to a broad application area, these protocols are easy of scaling to decagrams, while exhibiting exceptional substrate generality and functional group compatibility.3
[1] J. C. Siu, N. Fu, S. Lin, Acc. Chem. Res., 2020, 53, 547-560.
[2] R. Francke, R. D. Little ChemElectroChem, 2019, 6, 4373-4382.
[3] S. Patra, I. Mosiagin, R. Giri, T. Nauser, D. Katayev, Angew. Chem. Int. Ed., 2023, e202300533.