Surface Morphology and Interfacial Electric Fields of Inorganic Materials
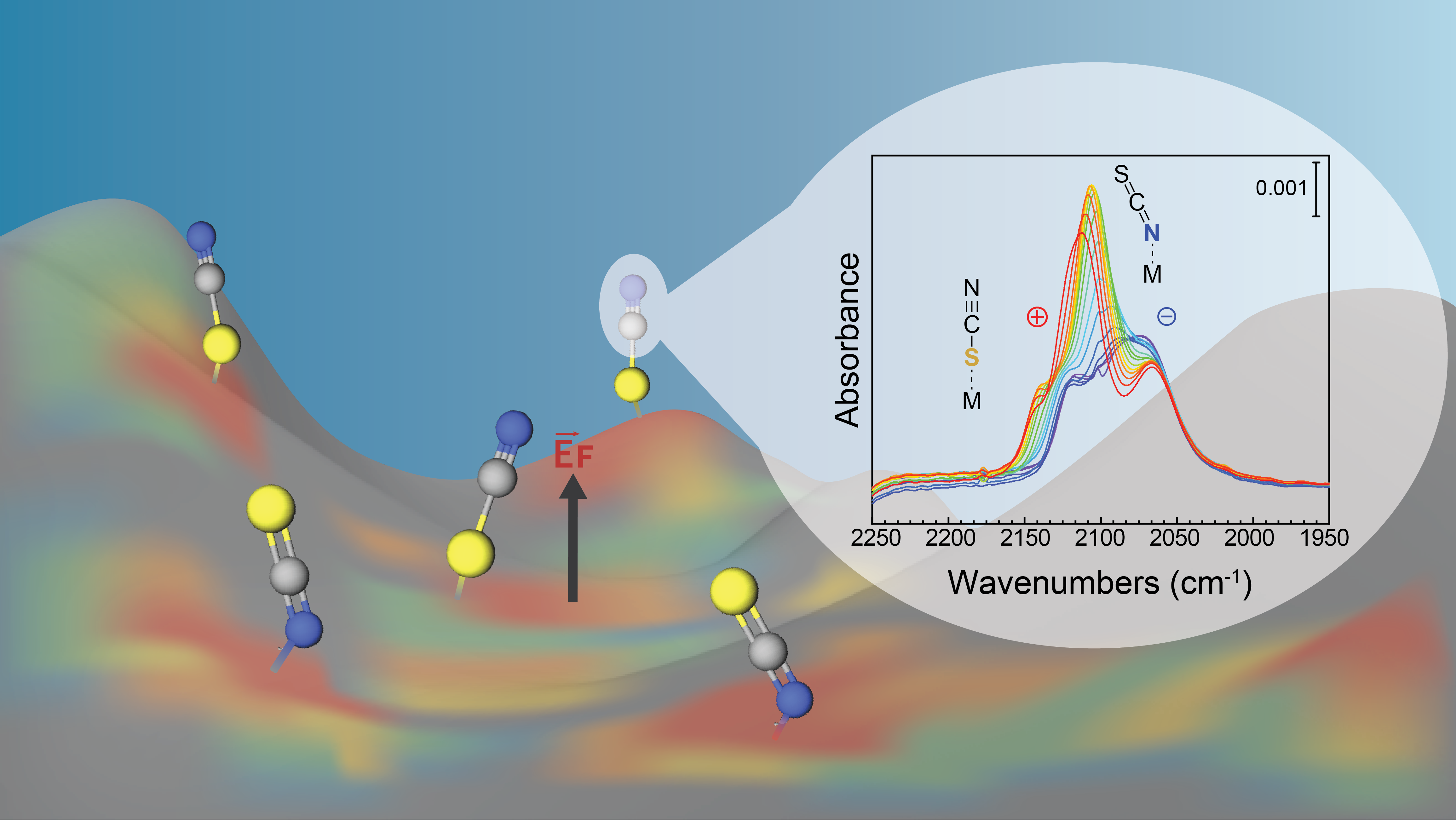
Sustainable energy conversion technologies often rely on an inorganic material as electrocatalyst. The activity and selectivity of such electrocatalysts critically depend on a complex interplay of the electric field at the solid-solution interface, the coordination of substrates and other solution components at the inorganic surface, the surface morphology that influences the diffusion of electrons and ions to the active surface sites, and other factors.1-7 However, these interfacial fundamentals remain elusive, in part because many conventional characterization methods are either not surface-specific or cannot be assessed under reaction conditions. Here, we aim to disentangle the relationship between surface morphology, chemical binding of solution components and electric field effects at the surface of inorganic material electrocatalysts by a spectro-electrochemical approach.
We employed in situ surface-enhanced infrared absorption spectro-electrochemistry (SEIRAS) to probe the interfacial chemistry of thin electrode films of inorganic materials having different surface morphologies as a function of an applied potential. A series of probe molecules and chemical surface modification strategies were combined to report on the presence or absence of pinholes and other defects at the surface, to provide insight into the nature of coordinative surface binding for both earth-abundant inorganic materials and noble metals, and to measure the electric field locally at the surface on molecular-length scales. By careful analysis of the infrared spectra, we provide first clues on the complex behavior of the polarized surfaces of inorganic materials and provide tools to analyze their potential relevance in catalysis.
[2] Murata, A.; Hori, Y. Bull. Chem. Soc. Jpn. 1991, 64, 123−127.
[3] Resasco, J.; Chen, L. D.; Clark, E.; Tsai, C.; Hahn, C.; Jaramillo, T. F.; Chan, K.; Bell, A. T. J. Am. Chem. Soc. 2017, 139, 11277−11287.
[4] Liu, M.; Pang, Y.; Zhang, B. et al. Nature 2016, 537, 382–386.
[5] Li, F.; Medvedeva, X.V.; Medvedev; J.J. et al. Nat Catal. 2021, 4, 479–487.
[6] Delley, M. F.; Nichols, E. M.; Mayer, J. M. J. Am. Chem. Soc. 2021, 143, 10778−10792.
[7] Delley, M. F.; Nichols, E. M.; Mayer, J. M. J. Phys. Chem. C 2022, 126, 8477–8488.