Synthesis and antimicrobial activity of rhenium tricarbonyl complexes bearing medically relevant ligands and selected by computer-aided design
For more than 30 years, no new class of antibiotics has reached the market, which has caused antimicrobial resistance (AMR) to increase in a concerning manner. Metal complexation of well-established drugs constitutes a promising strategy to overcome loss of medication sensitivity and induced resistance, as the coordinated derivatives of biologically active ligands have been known to show synergistic effect with certain metal cores [1]. The choice of the latter yet remains critical and, in particular, rhenium (Re) complexes feature interesting medical properties, especially because the final oxidation product of such species displays extremely low toxicity, comparable to the one of sodium chloride [2].
Examples of Re derivatives developed for antimicrobial purposes remain scarce in the literature. However, Mendes et al. recently studied the antibacterial mechanism of such a molecule (1) bearing the well-established clotrimazole ligand. They discovered that this promising complex inhibits cell wall synthesis by interfering in the MurG-mediated production of Lipid II from Lipid I (Figure 1A) [3].
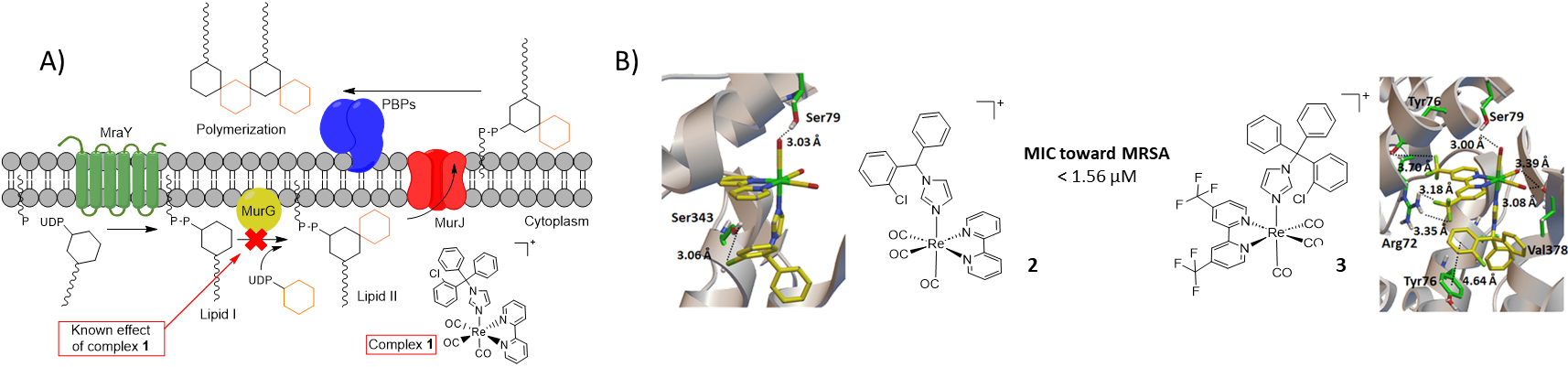
Figure 1. A) Peptidoglycan biosynthesis where the MurG-mediated reaction is inhibited by 1. B) Interaction maps of the absolute lowest energy poses of 2 and 3 in SpsB catalytic site.
Using computational methods, our group has been investigating similar Re-containing enzyme blockers in order to develop new efficient antibiotics [4,5]. This computer-aided strategy consists in performing docking calculations followed by synthesis and finally antimicrobial assessment of the candidates with best docking scores in the selected enzyme pocket. Considering the results obtained so far (see e.g. complexes 2 and 3, Figure 1B), we believe that this approach constitutes a viable strategy in the fight against AMR.
[2] Haase, A.A.; Bauer, E.B.; Kuhn, F.E.; Crans, D.C. Coord. Chem. Rev. 2019, 394, 135-161.
[3] Mendes, S.S.; Marques, J.; Mesterházy, E.; Straetener, J.; Arts, M.; Pissarro, T.; Reginold, J.; Berscheid, A.; Bornikoel, J.; Kluj, R.M.; et al. ACS Bio & Med Chem Au 2022, 2, 419-436.
[4] Schindler, K.; Cortat, Y.; Nedyalkova, M.; Crochet, A.; Lattuada, M.; Pavic, A.; Zobi, F. Pharmaceuticals 2022, 15, 1107.
[5] Cortat, Y.; Nedyalkova, M.; Schindler, K.; Kadakia, P.; Demirci, G.; Nasiri Sovari, S.; Crochet, A.; Salentinig, S.; Lattuada, M.; Steiner, O.M.; Zobi, F. Antibiotics 2023, 12, 619.