Selective olefin transfer hydrogenation of unsaturated carbonyls with ethanol using a PYA ruthenium(II) complex
Hydrogen production is one of the most polluting processes in chemistry generating about 900 million tons of CO2 each year, or more than 2% of world CO2 emissions.[1] Alcohols, applied in transfer hydrogenation reactions, are known alternatives to H2 that can be sourced from renewable feedstock. Historically, isopropanol has been widely investigated but is limited in terms of utilization, safety, and production. In this context, ethanol has found an increasing interest in the past few years, but its use is still limited by its ability to form stable carbonyl compounds[2] and side reactions such as α-alkylation under basic conditions.[3]
Here, we propose the use of pyridylidene-amide (PYA) complexes as efficient catalysts to propel ethanol-based transfer hydrogenation. PYAs are unique ligands featuring electronically flexible properties that can stabilize the metal center during a catalytic cycle and thus increase its activity and robustness in a variety of reactions, e.g. olefin and water oxidation (Fig. 1a).[4] Here, we will present a novel N,N-bidentate ruthenium(II) complex that is readily accessible with high stability towards air and moisture. Excellent activity and selectivity in the reduction of α,β-unsaturated compounds were obtained in mild conditions for a wide range of substrates (Fig. 1b). The use of ethanol as hydrogen source at room temperature combined with catalytic use of common laboratory base makes the system cost-effective and attractive for industrial and pharmaceutical applications.
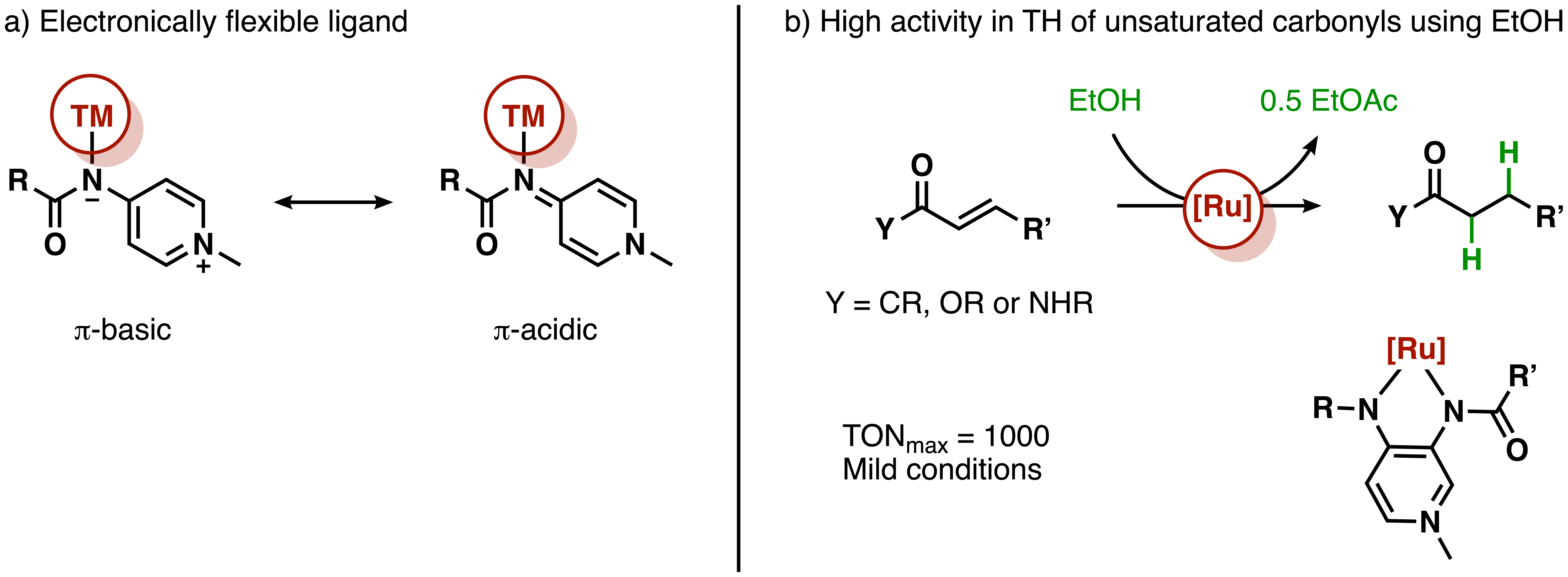
Figure 1.
[1] Davide Castelvecchi, Nature, 2022, 611, 440-443.
[2] Bruno N. Chaudret, David J. Cole-Hamilton, Ronald S. Nohr, Geoffrey Wilkinson, Journal of the Chemical Society, Dalton Transactions, 1977, 16, 1546-1557.
[3] Takashi Kuwahara, Takahide Fukuyama, Ilhyong Ryu, Organic Letters, 2012, 14, 4703-4705.
[4] Miquel Navarro, Mo Li, Stephan Bernhard, Martin Albrecht, Dalton Transactions, 2018, 47, 659-662; Kevin Salzmann, Segarra, C.; Albrecht, M. Angewandte Chemie International Edition, 2020, 59, 8932-8936.