Directional ionic bonds
Ionic bonds are the strongest type of noncovalent interactions and might reach energies comparable to covalent bonds.[1,2] While covalent bonds are inherently highly directional, ionic bonds lack such property due to the spherically-symmetrical nature of the electric field around simple ions.[3] We describe a strategy to impart the directionality to ionic bonds allowing them to acquire a predictable directional orientation.[4] Our design of directional ionic bonds includes the installation of a sterically demanding nonpolar hydrocarbon groups around the ions that leave the charged atoms exposed to one direction only. In this way, we minimize the charge separation and maximize the Coulomb attractive forces, while other relative orientations result in larger charge separations due to the steric repulsion between the shielding backbones.
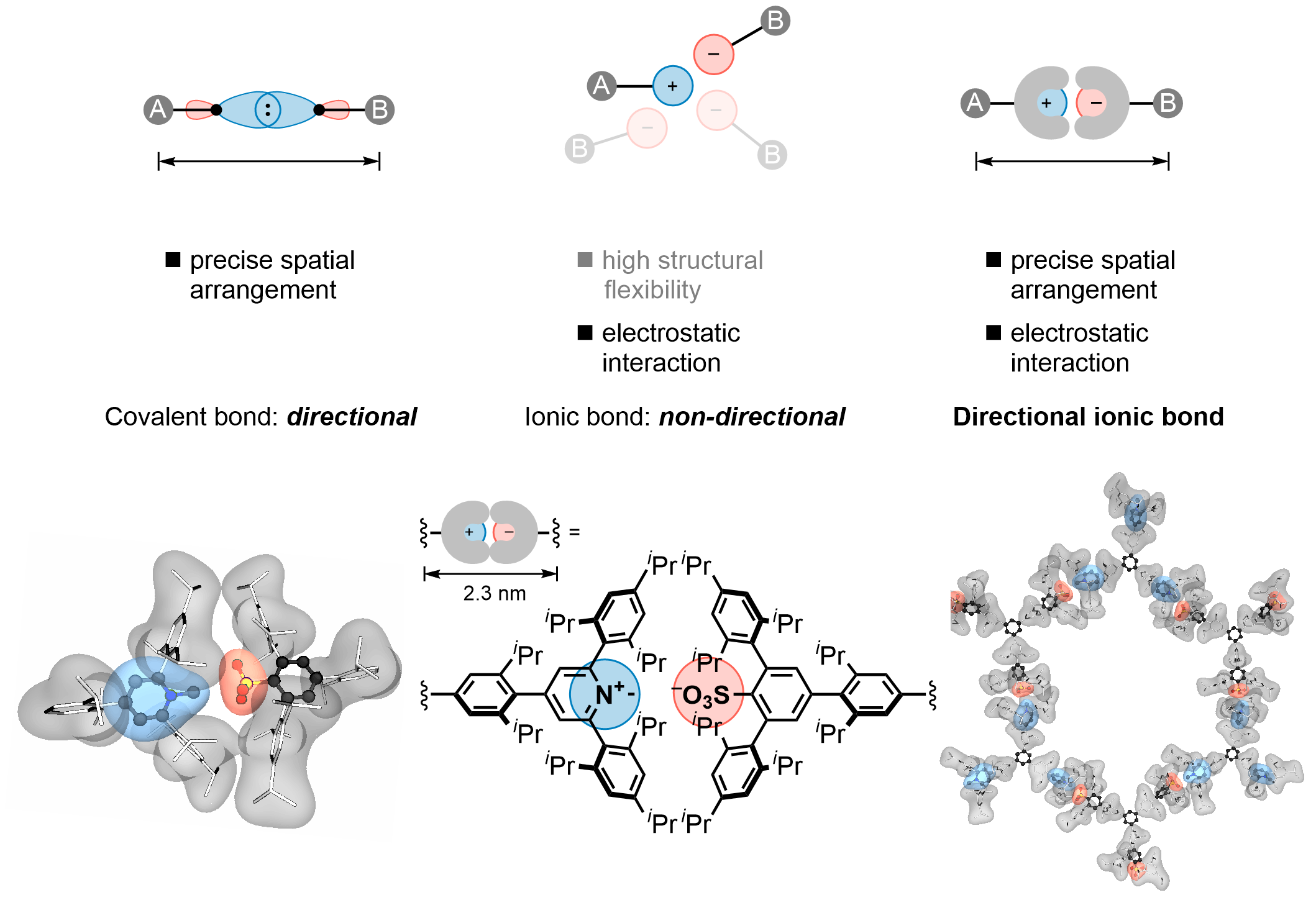
The generality of our concept is showcased with a series of N-methylpyridinium∙∙∙arylsulfonate ion pairs that possess different shielding groups around the charged moieties. Furthermore, multiple directional ionic bonds were utilized to build supramolecular systems at the nanoscale by the formation of a two-dimensional hexagonal lattice composed of six directional ionic bonds. Such ionically bonded framework is an example of a complementary strategy for the directional construction of organic materials by exploiting the otherwise nondirectional Coulomb interactions between two ions.
[2] Biedermann, F.; Schneider, H.-J., Chem. Rev., 2016, 116, 5216–5300.
[3] Faul, C. F. J.; Antonietti, M., Adv. Mater., 2003, 15, 673–683.
[4] Hutskalov, I.; Linden, A.; Čorić, I., J. Am. Chem. Soc., 2023, 145, 8291–8298.