Iodine atom transfer mediated radical addition - cyclization processes using alpha-boryl radicals
Since the pioneering work of Suzuki[1] and his discovery of cross coupling reactions, boron has emerged as an important element for organic synthesis. Moreover, boron is as well contained in active compounds and, until 2022, 5 of them were approved by the FDA[2]. It is thus important to develop new organic reactions which help synthesizing new boron containing compounds. We exploited the unique features of alpha-boryl radicals and their addition to unsaturated systems[3][4] to develop an easy, scalable and efficient reaction to access 1,5-iodoboronic esters which possess a cyclopentane scaffold via 5-exo-trig cyclization. The optimization of the conditions and a scope of the reaction will be presented. The new products can be derivatized using further post-functionalization. Our new methodology could be used for the synthesis of small natural products, such as in the case of iridolactones.
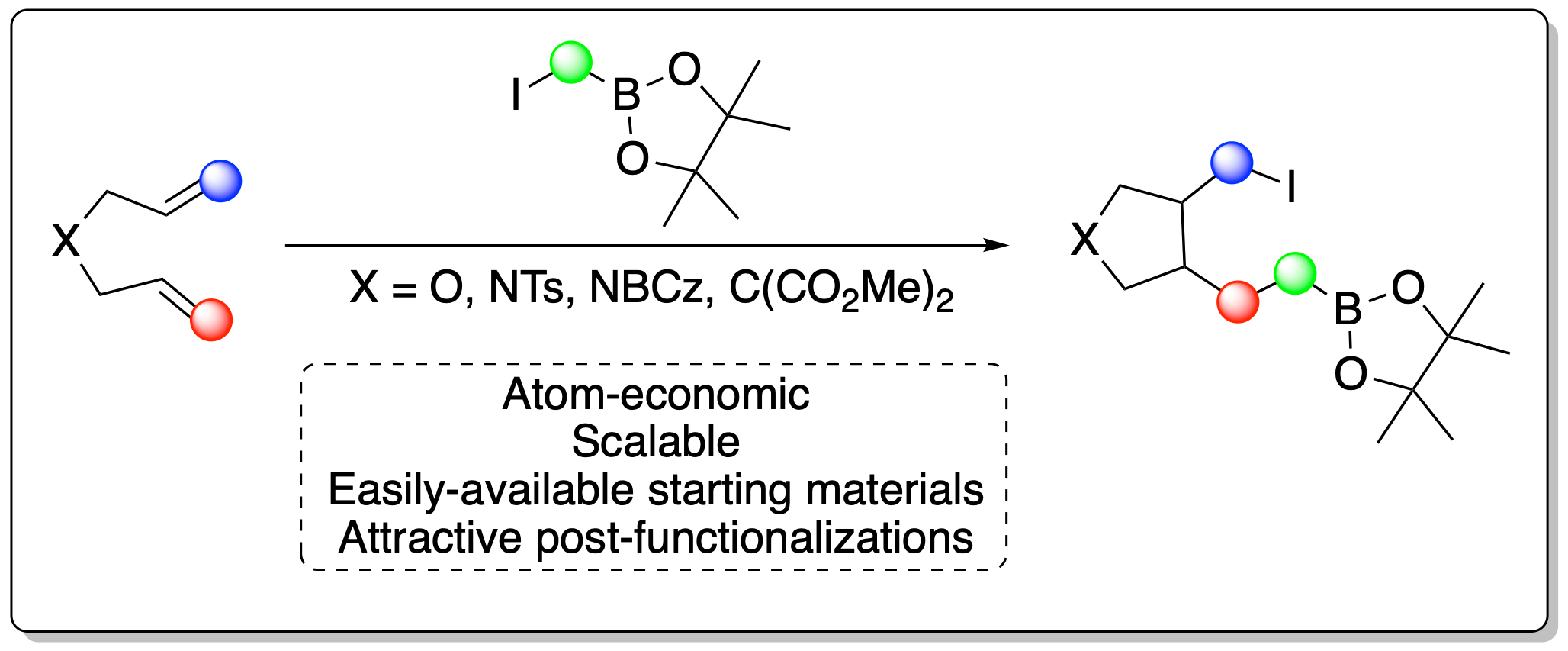
[2] K. Messner, B. Vuong, G. K. Tranmer, Pharmaceuticals 2022, 15, 264.
[3] Q. Huang, J. Michalland, S. Z. Zard, Angew. Chem. Int. Ed. 2019, 58, 16936–16942.
[4] N. D. C. Tappin, W. Michalska, S. Rohrbach, P. Renaud, Angew. Chem. Int. Ed. 2019, 58, 14240–14244.