Frustrated Ion Pair-Enabled Cross-Electrophile Coupling of Unactivated Alkyl Electrophiles
Cross‑electrophile coupling reactions have emerged as a powerful strategy for C–C bond formation. One of their advantages is that they avoid the need to prefunctionalize one of the coupling partners as a reactive organometallic reagent in contrast to traditional cross‑coupling reactions. They are most often catalyzed by transition metals and rely on an external reductant after every turnover. Due to the environmental impact of transition metals and economic considerations, the development of transition metal-free strategies is desirable. Despite progress in this area, the coupling of two unactivated C(sp3) reaction partners by such means has so far remained elusive.
We report a cross-electrophile coupling between two unactivated C(sp3) fragments in a transition metal-free protocol. Alkylphosphonium salts are reacted with alkyl halides in the presence of a hindered base as the sole reagent to give rise to the coupled products.[1]
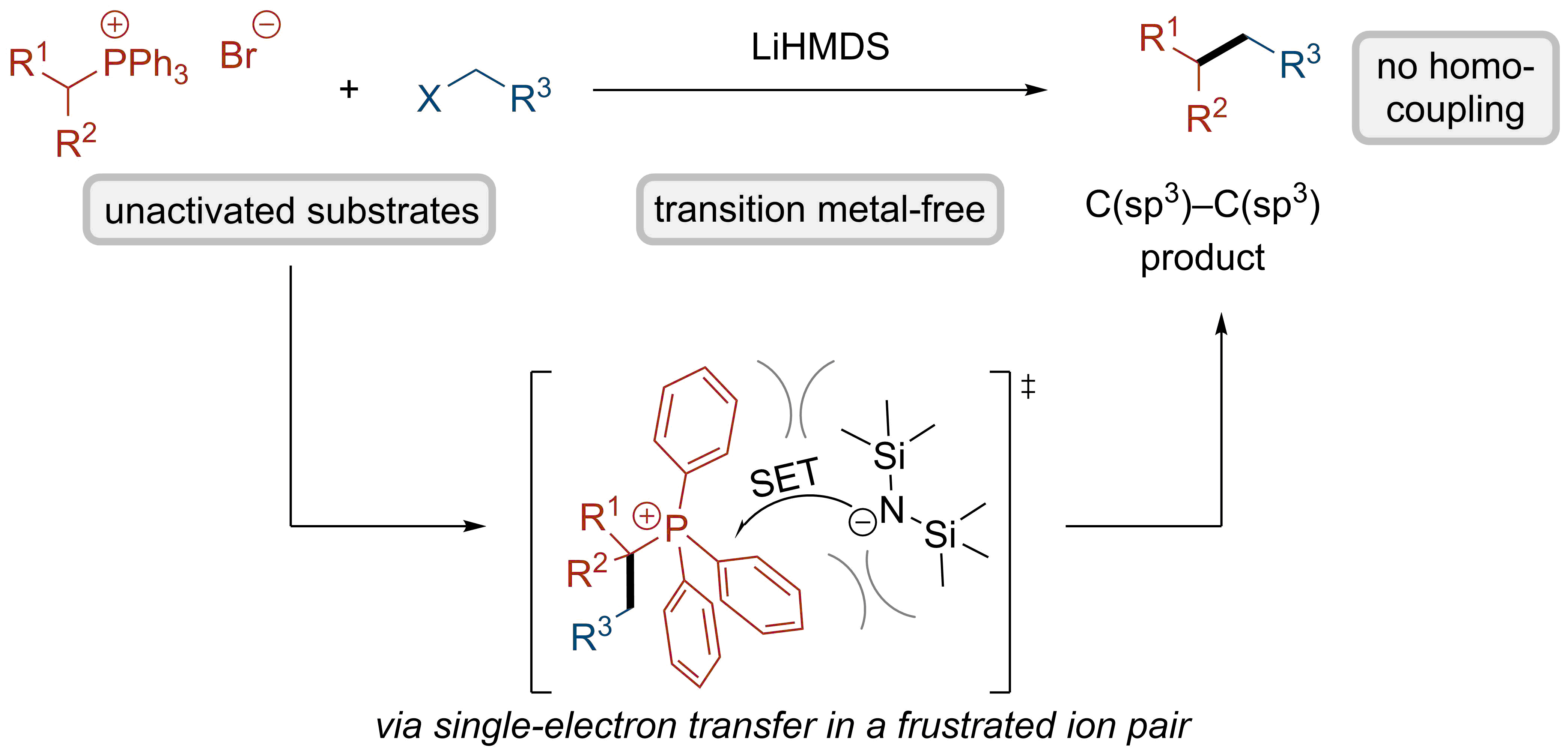
The reaction tolerates several moieties that would be challenging under transition metal-catalyzed conditions, demonstrating the advantages of the metal-free approach. Detailed experimental and computational mechanistic studies indicate that the reaction is enabled by a single-electron transfer event in a frustrated ion pair. This unusual reactivity could be leveraged to extend the reactivity beyond cross-electrophile coupling, highlighting its potential in organic synthesis.