Understanding enzyme-nanoparticle interactions during bioelectrocatalytic hydrogen formation
The demand for renewable fuels such as H2 continues to rise during the climate crisis. Metalloenzymes such as [FeFe]-hydrogenase offer an attractive option for electrocatalytic H2 evolution as they operate under mild conditions. To produce hydrogen electro-enzymatically, the biocatalyst can be immobilized on (and electronically wired to) an electrode surface, where a potential is applied to drive H+ reduction. However, these bioelectrocatalytic systems often suffer from reduced stability.
We recently reported a technique for the facile functionalization of carbon electrodes with an In:Sn oxide (ITO) nanostructured architecture, onto which [FeFe]-hydrogenase was directly immobilized.1 H2 production by the bioelectrode was recorded by cyclic voltammetry to high current densities of > 8 mA cm−2 at −0.8 V vs. SHE (pH 7), and maintained ~92 % of the original current density after 5 days of continuous potentiostatic operation. Interestingly, the current density for H2 production on ITO was > 38x higher than when [FeFe]-hydrogenase was immobilized on planar glassy carbon (GC). We hypothesize there may be stabilizing interactions between the enzyme and ITO surface which can account for this enhanced performance.
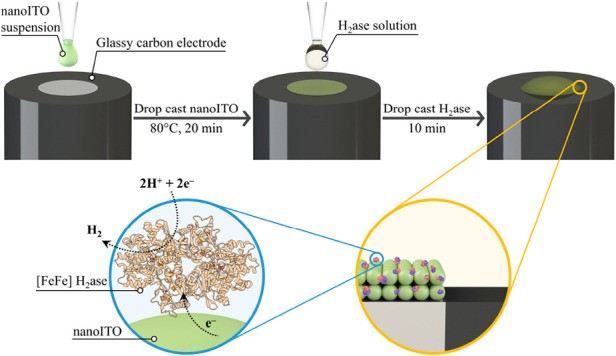
In this work, we further investigate properties of the ITO architecture which may contribute to the high performance of this bioelectrode. The electrocatalytic activity of the enzyme during H2 production is compared between nanostructured and planar ITO to understand if the nanostructured surface stabilizes the hydrogenase. The thickness of the ITO surface is varied in order to understand the impact on enzyme loading and performance. This is realized using a quartz crystal microbalance (QCM), coupled with electrochemistry, to following the adsorption process of the enzyme and to calculate its loading. We anticipate that these results can be utilized in combination with rotating ring disk electrochemistry to aid a deeper kinetic investigation of the bioelectrochemical system.
We anticipate that further investigation of the ITO architecture can aid in the application of this ‘nanoITO’ electrode in a combination with other (metallo)enzymes of interest to electroenzymatic biotechnologies which are harder to study electrochemically.