Alkali-Metal Silyl Zincates: Synthesis, Structure and Reactivity
The high natural abundance of silicon and its non-toxic nature are just some of this element’s advantages, strengthened by the popular use of organosilicon compounds within cross-coupling reactions.[1] Therefore, there stands a natural drive to easily access such useful organosilicon building blocks. While numerous pathways for the silylation of organic molecules have been designed using electrophilic silicon species (mainly using transition metals and/or harsh conditions), the use of nucleophilic silicon reagents is a lesser-employed alternative.[2] Current methods that have emerged within the past decade include nucleophilic addition or substitution reactions via silylmetalation,[3a] silylboration,[3b] defluorosilylation[3c] or nucleophilic silyl substitution[3d] using monometallic-silyl reagents. Despite these advances, a gap in the knowledge still exists with regards to (i) fundamental characterisation and understanding of these bimetallic reagents employed and (ii) the modus operandi which they follow.
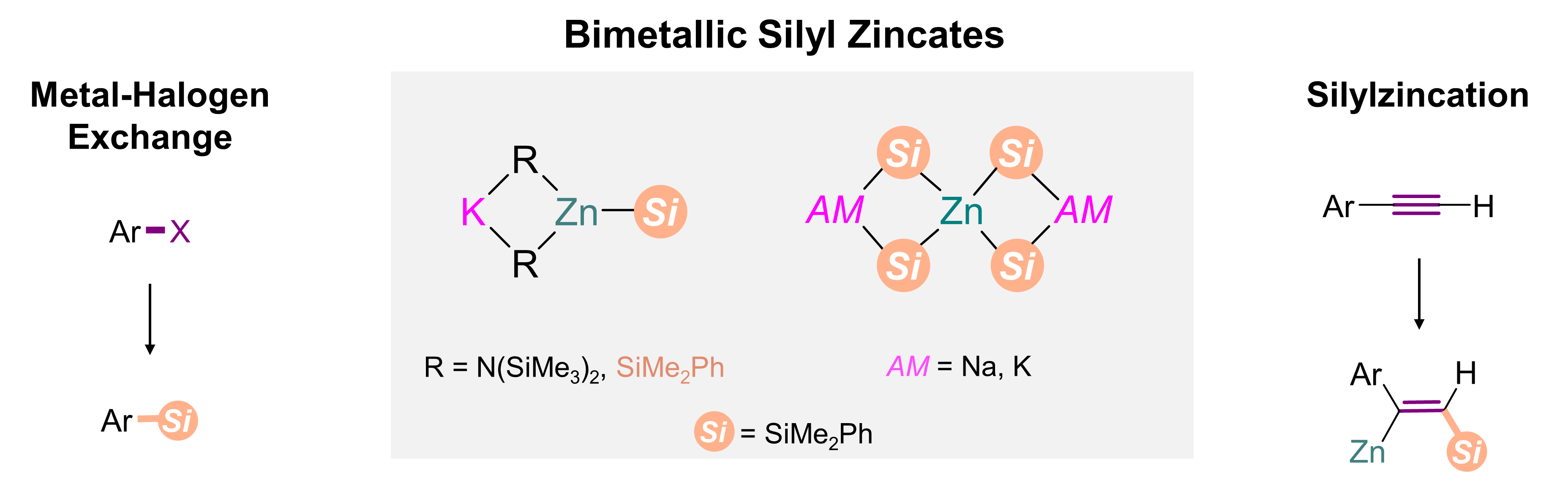
In an effort to bridge this gap, this work exploits the high reactivity of alkali-metals (e.g. K) combined with the better selectivity of a lower-polarity metal (Zn) as a platform for accessing novel, nucleophilic silyl complexes. The results presented include X-ray crystallographic characterisation of these bimetallic silyl reagents, and our preliminary understandings surrounding their nucleophilic capabilities in the direct silylation of haloarenes or the silylzincation of phenylacetylene.
[2] Takeshi Komiyama, Yasunori Minami, Tamejiro Hiyama, ACS Catal., 2017, 7, 631-651; Watchara Srimontree, Lin Guo, Magnus Rueping, Chem.-Eur. J., 2020, 26, 423-427.
[3] (a) Shinki Nakamura, Mitsuhiro Yonehara, Masanobu Uchiyama, Chem. Eur. J., 2008, 14, 1068-1078. (b) Yiting Gu, Yaya Duan, Yangyang Shen, Ruben Martin, Angew. Chem. Int. Ed., 2020, 59, 2061-2065. (c) Greg Coates, Hui Yee Tan, Carolin Kalff, Andrew J. P. White, Mark R. Crimmin, Angew. Chem. Int. Ed., 2019, 58, 12514-12518; d) Eiji Yamamoto, Satoshi Ukigai, Hajime Ito, Synlett, 2017, 28, 2460-2464.