Organocatalytic Synthesis of Triflones Bearing Two Non-Adjacent Stereogenic Centers
The trifluoromethylsulfonyl (SO2CF3, triflyl) group is an intriguing functional group for its properties and unique reactivity since it combines the well-established transformations of alkyl and aryl sulfones with reactivity exclusive to the triflyl group.[1] Methods to access triflones are therefore enabling tools for organic synthesis. However, strategies to obtain chiral triflones are limited.[2]
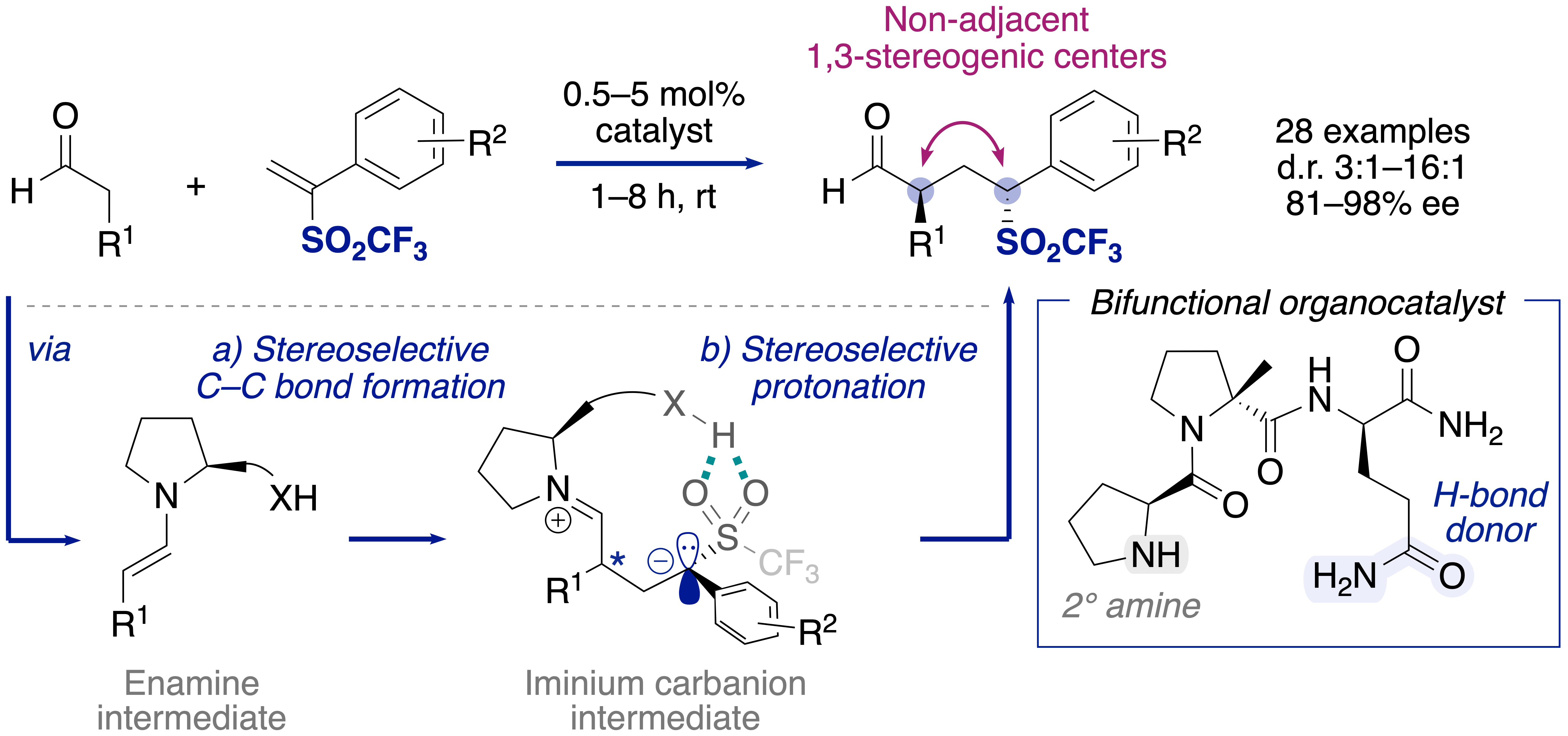
We developed an efficient organocatalytic synthesis of chiral triflones using α-substituted vinyl triflones, building blocks previously unexplored in asymmetric catalysis.[3] This peptide-catalyzed conjugate addition provides a broad range of γ-triflylaldehydes with two non-adjacent stereogenic centers in high yields and stereoselectivities. The reaction proceeds at a low catalyst loading of 0.5–5 mol% and tolerates a variety of functional groups, including acetal, ester, or ketone moieties. We show that a bifunctional peptide catalyst is crucial for high diastereo- and enantioselectivity through stereocontrol of the conjugate addition and protonation steps. Furthermore, straightforward derivatization of the products into 1,3-disubstituted heterocycles, including δ-sultones, γ-lactones, and pyrrolidines, highlights the versatility of γ-triflylaldehydes. Our results showcase the value of vinyl triflones for organic synthesis and open new possibilities to access chiral compounds with 1,3-stereogenic centers from α-substituted Michael acceptors.
[2] X. H. Xu, K. Matsuzaki, N. Shibata, Chem. Rev. 2015, 115, 731–764.
[3] A. Budinská, H. Wennemers, Angew. Chem. Int. Ed. 2023, e202300537.