15N NMR Anisotropy on Transition Metal Imido Complexes: Link between Spectroscopy and Reactivity
Imido ligands are one of the most fundamental ingredients in organometallic chemistry serving as supporting ligand, which can be typically found in Schrock-type olefin metathesis catalysts, and as a reactive site which facilitates numerous stoichiometric- and catalytic transformations such as cycloaddition with unsaturated bonds, nitrene transfer or C-H bond activation. Despite the diverse reactivity found among transition metal imido complexes across the periodic table, no general reactivity descriptor has been uncovered. In this context, solid-state NMR (ssNMR) has recently emerged as a powerful tool to link the spectroscopic signature, i. e. chemical shift tensors (CSTs), of specific nuclei to their electronic structures, which can be further tied with the reactivity.1 In this work, we uncover the electronic structures of transition metal imido complexes using solid-state NMR augmented with DFT calculations and establish a spectroscopy-reactivity relationship.
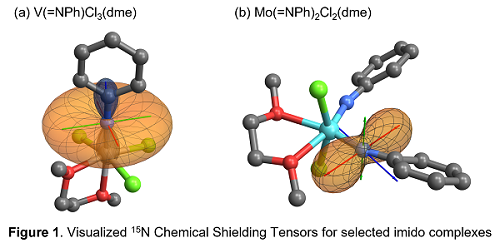
The experimentally-collected 15N ssNMR spectrum of the group 5 mono-imido species V(=15NPh)Cl3(dme), which has inert imido ligand, revealed an axial spectral pattern (d11 ≈ d22) while the highly reactive group 6 bis(imido) species Mo(=15NPh)Cl2(dme) exhibited an anisotropic spectrum (d11 ≠ d22). Natural Chemical Shift (NCS) analysis anchored on the experimentally-determined chemical shift anisotropy revealed the dominant and highly asymmetric development of p-contribution on the CSTs in Mo(=NPh)2Cl2(dme), while s-bond only plays a marginal role. This contrasts with the symmetric distribution of s- and p-orbitals in the unreactive V(=NPh)Cl3(dme), in which the s(V-N) orbital dominates. The highly developed contribution from the p(Mo-N) orbital in Mo(=NPh)2Cl2(dme) points out its high-lying nature. Visualization of the molecular orbitals revealed the competitive p-interaction between two imido ligands sharing the same orbital, which induces an increase HOMO energy and reactivity. Such scenario further supports the so-called concept of the “p-loading effect”,2 which is often referred as an activation strategy for imido complexes. The thus-obtained results show that 15N chemical shift tensors are useful probe of reactivity for imido complexes.
[2] Lapina, O. B.; Mats'ko, M. A.; Mikenas, T. B.; Zakharov, V. A.; Paukshtis, E. A.; Khabibulin, D. F.; Sobolev, A. P. Kinetics and Catalysis, 2001, 42, 553–560.