CO2 Hydrogenation to Methyl Formate using CO2 as the Sole Carbon Source
The atmospheric concentration of CO2 is still increasing, despite international efforts to curb emissions. The removal of CO2 from the atmosphere is a high-cost endeavour, necessitating economic incentives for carbon capture. More importantly, the use of the near-unlimited C1 resource of atmospheric CO2 as a building block for process chemicals is essential to a sustainable future.1 We have developed efficient catalysts for the hydrogenation of CO2 to formic acid (FA) and methanol. Additionally, we have combined these C1 building blocks into methyl formate (MF) in one system. Our catalysts are based on the encapsulation of noble metal complexes and sub-nanoparticles in the pores of zeolites, leveraging the acidity of the support and the principle of nanoconfinement to accelerate these multi-step reactions.
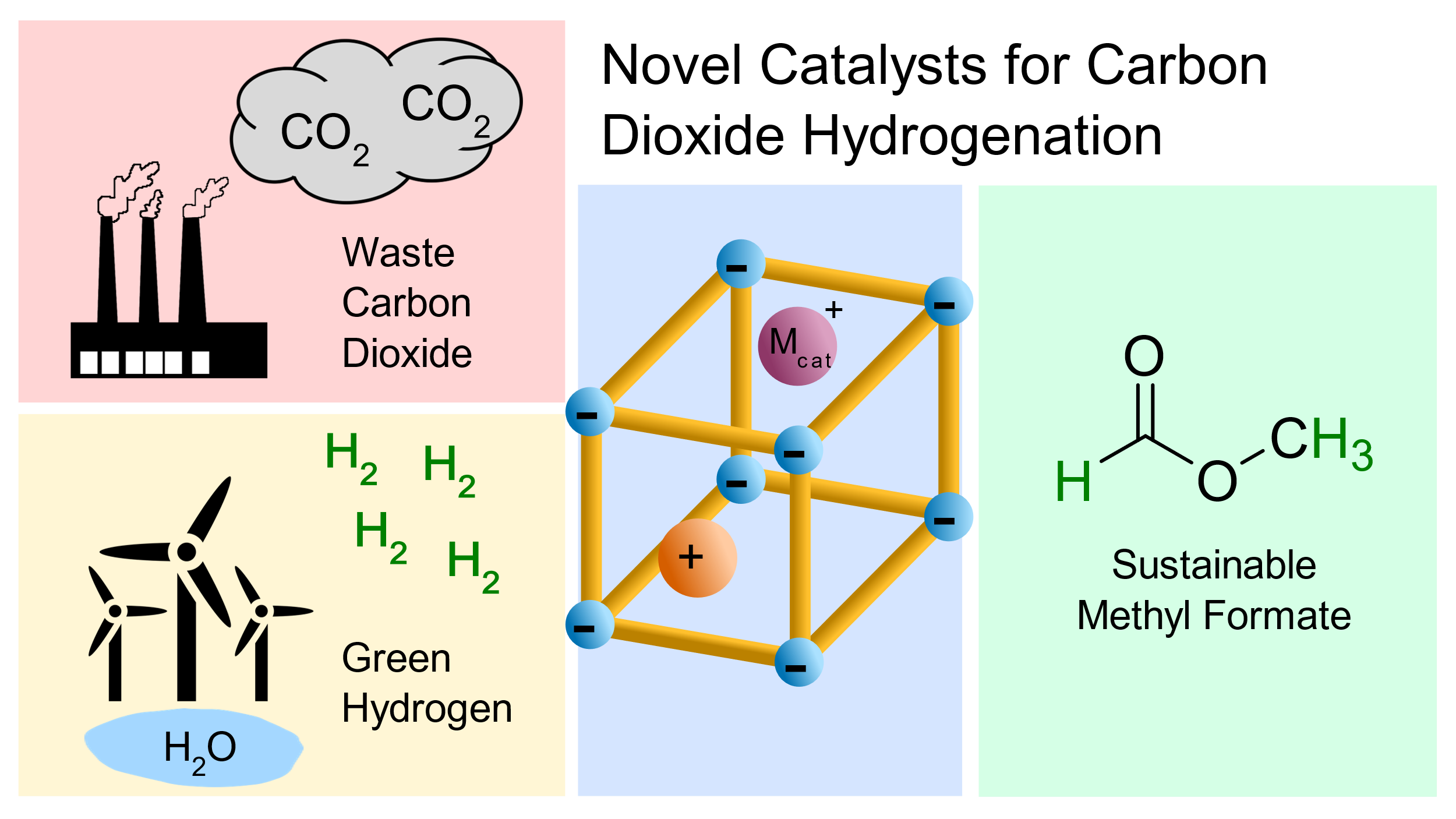
A Ru-based catalyst was developed for the hydrogenation of CO2 to FA, and simultaneously facilitates fast esterification to MF (TOF > 100 h-1), comparable to similar catalysts which operate at higher temperatures and pressures.2,3 A second series of catalysts, both from the literature and developed in our lab, are capable of CO2 and FA hydrogenation to MeOH under the applied conditions. Combining two appropriate catalysts, MF in which both C atoms derive from CO2 is formed. In the presence of a methoxide additive, the MF can be decarbonylated to give high CO concentrations (>20%) at a much lower temperature than traditional reverse water gas shift reactions.4 The structures of these catalysts will be discussed, and the reaction mechanisms will be explored to understand the key catalyst features which enable their reactivity. The present work allows the formation of three major C1 synthons widely used in industry using CO2 under relatively mild conditions. An outlook on coupling CO2 hydrogenation with C-C bond-forming reactions will be described, as we progress towards the sustainable and selective synthesis of C2+ products from atmospheric carbon.
[2] Yu, K. M. K.; Yeung, C. M. Y.; Tsang, S. C. J. Am. Chem. Soc. 2007, 129 (20), 6360–6361.
[3] Corral-Pérez, J. J.; Copéret, C.; Urakawa, A. J. Catal. 2019, 380, 153–160.
[4] González-Castaño, M.; Dorneanu, B.; Arellano-García, H. React. Chem. Eng. 2021, 6 (6), 954–976.