Synthesis and Reactivity of a Stable Organoazide Iron Complex and its Relevance to C–H Bond Amination
The formation of C–N bonds is of paramount importance for synthesis of pharmaceuticals, agrochemicals and natural products.[1] Complexes with organic azides are critical precursors for the formation of nitrene systems en route to the direct C–H amination, forming C–N bonds very efficiently and sustainably. Despite their relevance, first-row transition metals with α-organoazide coordinated are extremely rare,[2,3] and they have been elusive so far for iron, even though iron complexes are by far the most active C–H amination catalysts with organic azides.[4-6]
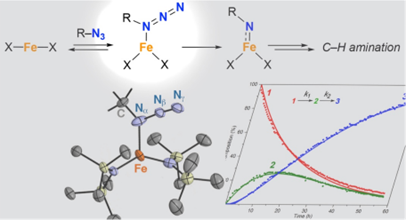
In this contribution we will show the first example of the full characterization of such an organoazide iron complex. We will demonstrate the further reactivity to a transient nitrene intermediate and discuss reactivity of the azide both in solution and in crystallo. The characterization of both these intermediates is of paramount importance for understanding the catalytic C–H amination reaction and for designing new and improved catalytic systems.
[2] Lauren N. Grant, Maria E. Carroll, Patrick J. Carroll, Daniel J. Mindiola, Inorg. Chem., 2016, 55, 7997–8002.
[3] Yunjung Baek, Anuvab Das, Shao-Liang Zheng, Joseph H. Reibenspies, David C. Powers, Theodore A. Betley, J. Am. Chem. Soc., 2020, 142, 11232–11243.
[4] Wowa Stroek, Martin Keilwerth, Daniel M. Pividori, Karsten Meyer, Martin Albrecht, J. Am. Chem. Soc., 2021, 143, 20157–20165.
[5] Wowa Stroek, Lilian Hoareau, Martin Albrecht, Catal. Sci. Technol. 2023, 13, 958-962
[6] Wowa Stroek, Martin Albrecht, Chem. Sci. 2023, 14, 2849-2859