Behavior of silver(I) ion binding with peptides inspired from SilE protein
Since many years, silver has been used in medicine for its antimicrobial properties.[1] However, as for antibiotics, bacteria can develop a certain resistance or tolerance to the antimicrobial action of silver.[2] For the Salmonella Typhimurium, this silver resistance is based on a silver efflux pump, composed of eight proteins which act together to export silver(I) ions, named Sil system.[2] While most of these proteins could be attributed in analogy to the copper efflux system Cus, the SilE protein is unique and proposed to act like a silver sponge.[2,3] SilE is composed by 143 amino acids (aa) and contains many histidine (His, H) and methionine (Met, M) residues, which are able to bind silver(I) ions.[4] The study of HXXM tetrapeptides has shown that the nature of the aa “X” influences the association binding constant log(Kass).[4]
Based on these observations and in order to gain a more basic understanding of silver ion binding in peptides and proteins, HXXM / MXXH / HXXH / MXXM tetrapeptides were synthesized and studied for their interactions with Ag+. Differences and similarities in the coordination behavior between HXXH and MXXM, but also HXXM and MXXH were noticed. Some first trends will be presented in this work.
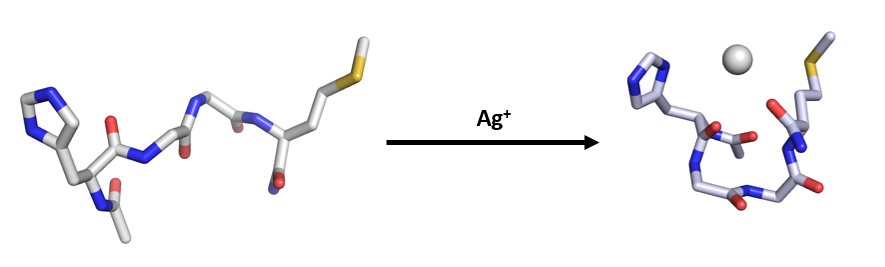
Fig. 1 : Folding of HXXM upon coordination with Ag+
[2] Simon Silver, FEMS Microbiol. Rev., 2003, 27, 341–353.
[3] Karishma R. Asiani, Huw Williams, Louise Bird, Matthew Jenner, Mark S. Searle, Jon L. Hobman, David J. Scott, Panos Soultanas, Mol Microbiol., 2016, 101, 731-742.
[4] Valentin Chabert, Maggy Hologne, Olivier Sénèque, Aurélien Crochet, Olivier Walker, Katharina M. Fromm, Chem. Commun., 2017, 53, 6105-6108.