Surfactant-driven Strategies for Sustainable C–H Activation: Progressing Towards Mild Reaction Conditions
Sustainability has become a top priority for every chemistry practitioner! Considering the development of a new reaction or that of a chemical process, sustainability, waste and energy minimization have become highly significant. The urgency has been further reenforced with the potential for a ban of several reprotoxic polar aprotic solvents such as DMF and NMP through the REACH regulation.1 Alternatives for such reaction media are indispensable. In the last decade, micellar conditions in bulk water have emerged as promising alternatives for several transformations such as a variety of cross-couplings or amide bond formation. In clear contrast, only a handful of examples emerged for C–H activations under micellar conditions, and the rare examples often require high temperatures.2
Our research focuses on the development of new catalytic systems for mild C–H activations occurring under micellar conditions. Two approaches are envisioned: 1) the careful design of additives to commercially available surfactants3 or 2) the implementation of novel designer surfactants, able to facilitate challenging C–H activations at ambient temperature.4
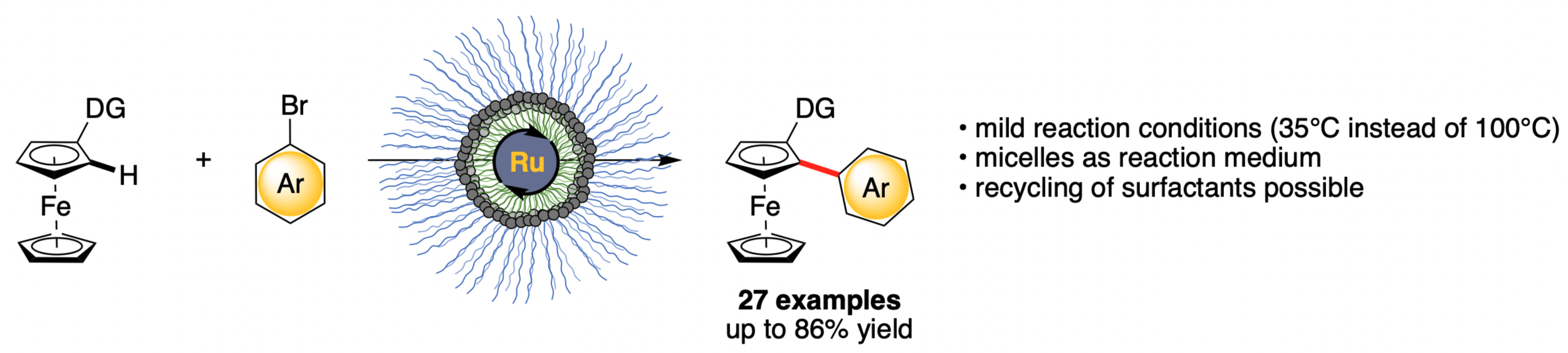
Remarkably, with newly designed surfactant obtained via installation of an additional ligand at the core of a commercially available surfactant in hand, we were able to lower the reaction temperature for the ruthenium C–H arylation of ferrocenes from 100°C to 35°C. Our conditions have shown to tolerate a broad spectrum of functional groups with yields up to 86% and a high chemoselectivity, enabling the late-stage functionalization of active pharmaceutical ingredients and natural products.
[2] P. Hauk, J. Wencel-Delord, L. Ackermann, P. Walde, F. Gallou, Curr. Opin. Colloid Interface Sci. 2021, 56, 101506.
[3] P. Hauk, V. Mazan, F. Gallou, J. Wencel-Delord, Manuscript under revision.
[4] P. Hauk, S. Trienes, T. Oyama, P. Vana, M. Andersson, J. Wencel-Delord, F. Gallou, L. Ackermann, Manuscript under revision.