The Photo Transformation of C1 Compounds; Methanol, Formaldehyde and Formic Acid via Natural Water Components
The photochemical transformation of naturally occurring organic molecules in the aquatic environment is an essential component of element cycling processes and important in ecosystem function. Photochemistry is not only involved in the buildup of organic molecules through photosynthesis in these systems, but also in their degradation. The degradation of dissolved organic matter (DOM) via photoirradiation can lead to an increase in biologically labile DOM (lower molecular weight components) and can thus provide a key source of growth substrates for naturally occurring microbial communities at the base of the food chain. Despite decades of environmental photochemistry research, we still do not understand the processes involved in the photoproduction and phototransformation of simple, ubiquitous compounds, such as methanol and other lower molecular weight acids in natural waters. Without deeper mechanistic insight, it is difficult to explain the reasons for these variations and how they may change, for instance, due to climate change.
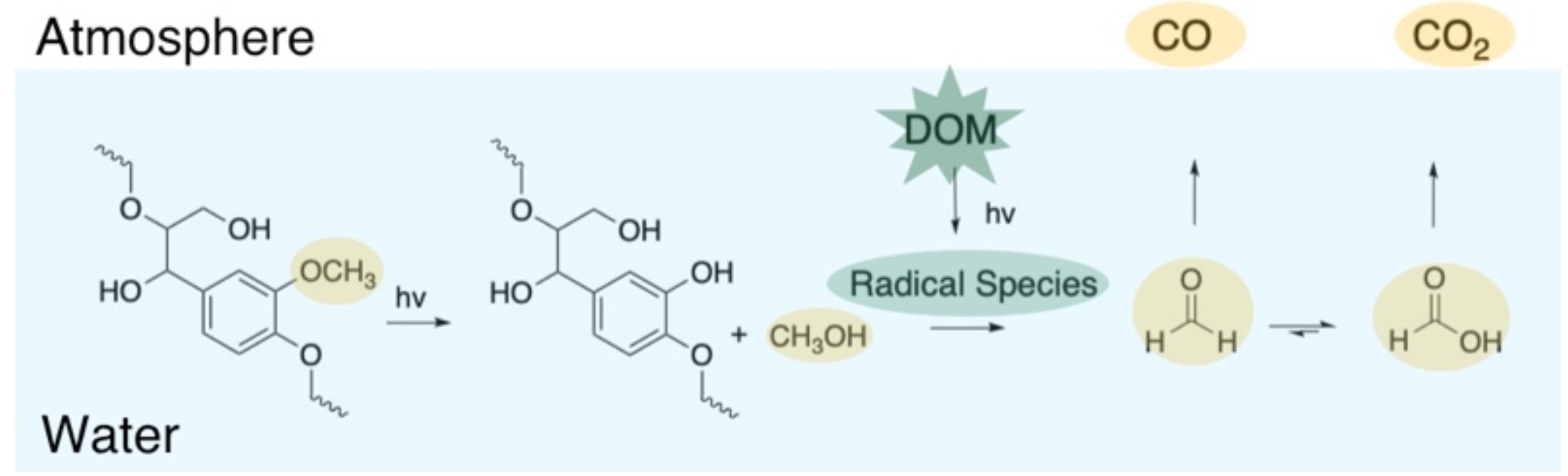
Previous research has demonstrated a link between the irradiation of sea water/DOM and observed the production of carbon monoxide and lower molecular weight acids. Our group further investigated this feature by irradiating a variety of lignin monomers to try and observe potential patterns of CO production and relating it to their chemical structure. We found that upon irradiation of lignin monomers in MQ water, the system produced methanol and CO, and the amount of these products was dependent on the presence of a methoxy group (observed in figure above) on the ring. Although a question remained, how did methanol convert to CO in a system only containing a lignin monomer and MQ water, and how may this represent a photochemical implication for these small compounds in environmental systems.
Upon irradiation of methanol and DOM over the period of 48 hours, we observed four key products; formaldehyde, formic acid, CO and CO2. The order in which they appeared gave us insight on the pathway and intermediates of complete or partial mineralization of methanol in potential aquatic environments. We observe that methanol reacts with OH radical as predicted whereas formic acid and formaldehyde are more sensitive to singlet oxygen. We also were able to observe different rates of CO/CO2 production during the experiments which helped us relate which products could be pre-cursors for what could end up in the atmosphere. We showed that formate oxidation led to production of CO2 when irradiated with DOM, whereas formaldehyde was the precursor to both CO and formate. These findings will be used to elaborate photochemical models of carbon cycling. We believe the transformations of C1 compounds play an important role in the photomineralization of C-substrates in the upper, well-lit zones of aquatic ecosystems.