Fast Viral Dynamics Revealed by Microsecond Time-Resolved Cryo-EM
Observing proteins as they perform their tasks has largely remained elusive, which has left our understanding of protein function fundamentally incomplete. To enable such observations, we have recently proposed a novel technique that improves the time resolution of cryo-electron microscopy (cryo EM) to microseconds [1-5]. We demonstrate for the first time that microsecond time-resolved cryo-EM enables observations of fast protein dynamics [6]. We use our approach to elucidate the capsid mechanics of cowpea chlorotic mottle virus (CCMV), whose large-amplitude motions play a crucial role in the viral life cycle [6]. We observe that a pH jump causes the extended configuration of the capsid to contract on the microsecond timescale. While this is a concerted process, the motions of the capsid proteins involve different timescales, leading to a curved reaction path.
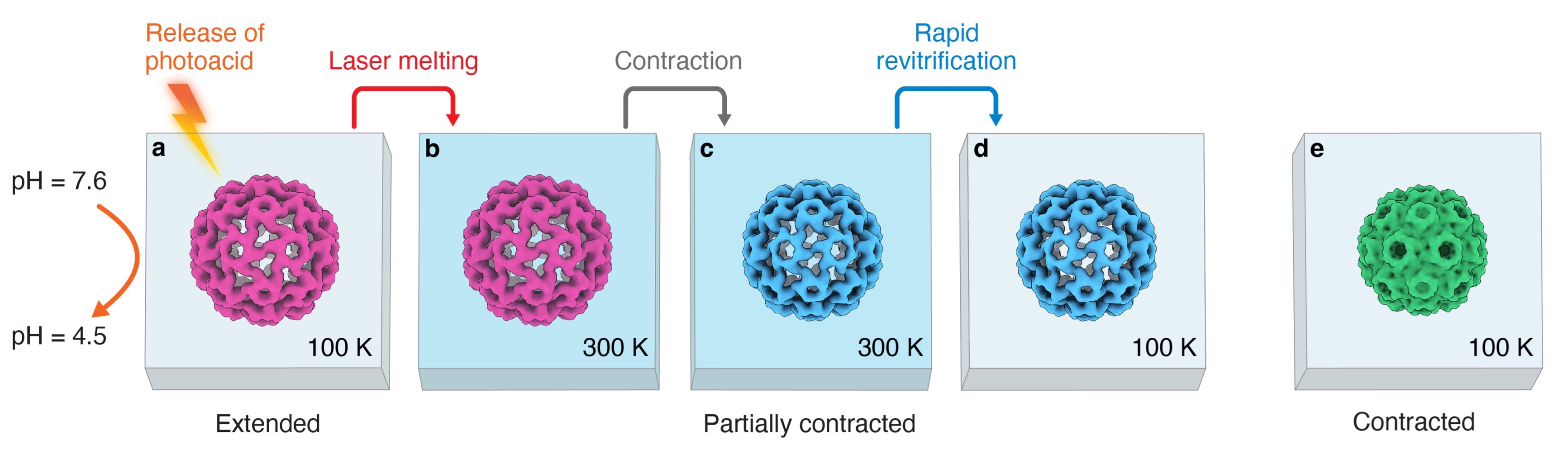
Fig. 1. Microsecond time-resolved cryo-EM of the CCMV contraction — experimental concept.
[1] J. M. Voss, O. F. Harder, P. K. Olshin, M. Drabbels, U. J. Lorenz, Struct. Dyn., 2021, 8, 054302
[2] J. M. Voss, O. F. Harder, P. K. Olshin, M. Drabbels, U. J. Lorenz, Chem. Phys. Lett., 2021, 778, 138812
[3] O. F. Harder, J. M. Voss, P. K. Olshin, M. Drabbels, U. J. Lorenz, Acta Crystallogr. Sect D. Struct. Biol., 2022, 78, 883–889
[4] G. Bongiovanni, O. F. Harder, M. Drabbels, U. J. Lorenz, Front. Mol. Biosci., 2022, 9, 1044509
[5] G. Bongiovanni, O. F. Harder, J. M. Voss, M. Drabbels, U. J. Lorenz, Acta Crystallogr. Sect D. Struct. Biol., 2023, 79, 473-478
[6] Oliver F. Harder, Sarah V. Barrass, Marcel Drabbels, Ulrich J. Lorenz, bioRxiv, 2023, 19.536710