Catalyst Control over Threefold Stereogenicity: C–S Atropisomeric Sulfones
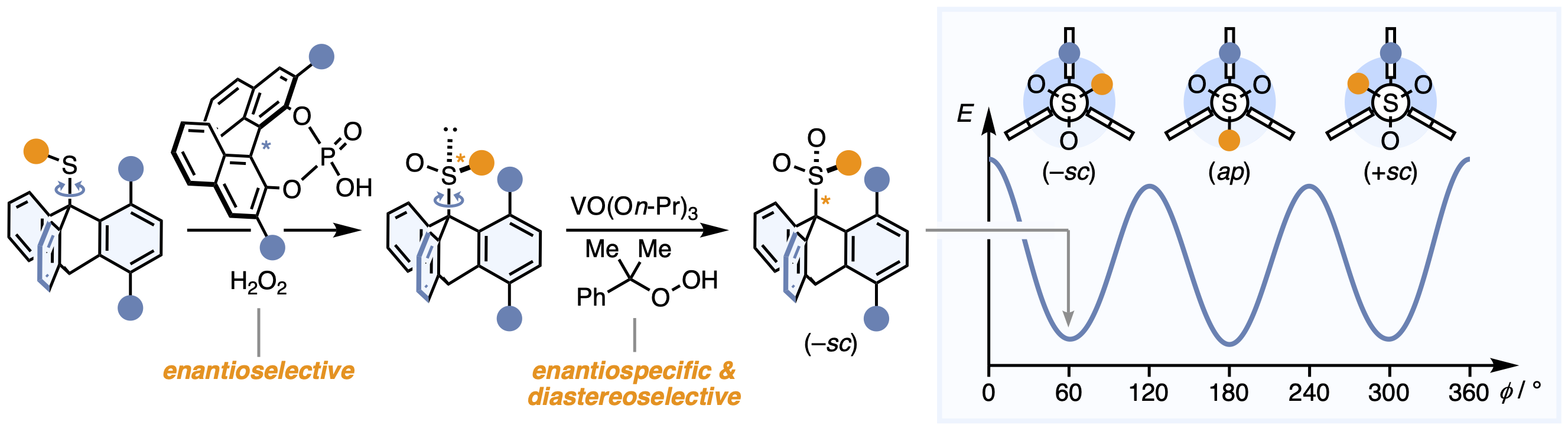
Numerous catalytic methods facilitate the stereoselective synthesis of atropisomers with rotationally restricted C–C, C–N, N–N and C–B single bonds between two planar moieties.[1] In contrast to these systems with two enantiomeric stereochemical states, catalyst control over atropisomers with more than two stereoisomers arising from one stereogenic axis was not accomplished until recently.[2] The Ōki triptycyl sulfones[3] exist in the form of three configurationally stable rotamers, namely the enantiomeric (±sc)-isomers and the symmetrical (ap)-conformer, which result from a rotationally restricted C–S bond connecting two tetrahedral fragments. We were able to stereoselectively access these systems by the catalyst-controlled oxidation of rotationally dynamic thioethers yielding enantioenriched sulfoxides, which subsequently were oxidized to the respective (sc)-sulfones.[4] While a chiral phosphoric acid catalyst defined the configuration of the sulfoxide stereocenter,[5] VO(On-Pr)3 in combination with cumene hydroperoxide rendered the second oxidation to the atropisomeric sulfones with threefold stereogenicity a highly enantiospecific and diastereoselective process. Using this strategy, the (–sc)-sulfones were obtained in high degrees of stereoisomeric enrichment with selectivities of up to 94:6:<1 (–sc):(+sc):(ap), representing the to the best of our knowledge first example of catalyst control over C–S atropisomerism. Moreover, by choosing distinct reaction conditions, the diastereoselectivity of the second oxidation step could be directed towards the (ap)-sulfone making all three stereoisomeric states selectively accessible under catalyst control.
[2] X. Wu, R. M. Witzig, R. Beaud, C. Fischer, D. Häussinger, C. Sparr, Nat. Catal. 2021, 4, 457–462.
[3] N. Nakamura, M. Ōki, Chem. Lett. 1984, 143–146.
[4] T. A. Schmidt, S. Schumann, A. Ostertag, C. Sparr, Angew. Chem. Int. Ed. 2023, 62, e202302084.
[5] Z.-M. Liu, H. Zhao, M.-Q. Li, Y.-B. Lan, Q.-B. Yao, J.-C.Tao, X.-W. Wang, Adv. Synth. Catal. 2012, 354, 1012–1022.