Comparison of the Protective Effect of Surfactants on Antibody Stability Against Different Interfaces
Maintaining the structural integrity of therapeutic proteins during their lifecycle is crucial for their efficacy and safety. However, interactions with various interfaces can compromise protein stability and lead to aggregation. During development, protein molecules and buffer composition must be optimized to ensure favorable physicochemical properties, but assays that systematically probe interfacial stability are lacking. To address this challenge, we have developed and applied a highly controlled nanoparticle-based method based on realistic and model interfaces to evaluate the stability of four monoclonal antibodies in the presence and absence of commonly used surfactants.
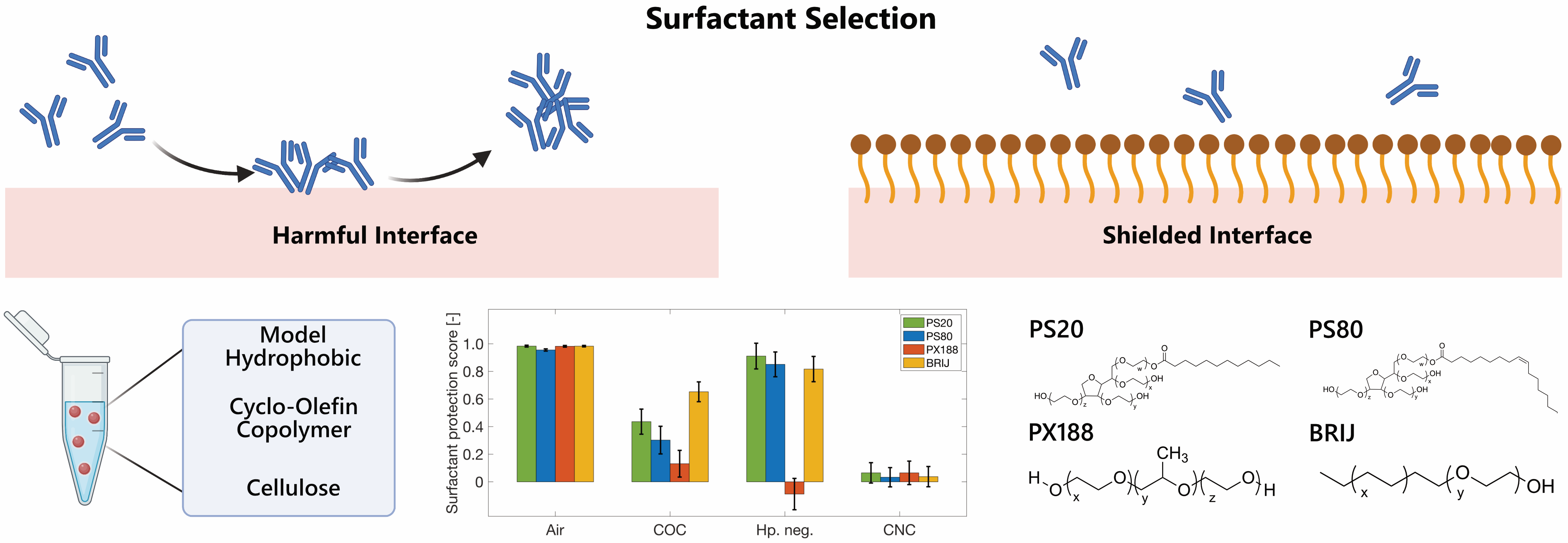
While all the surfactants demonstrated stabilizing effects against the hydrophobic air-water interface, none of them could safeguard the antibodies from hydrophilic charged cellulose. Additionally, polysorbates and Brij 35 provided a certain level of stability against COC and the model hydrophobic interface, while Poloxamer 188 had minimal impact on stability across these interfaces.
By rapidly evaluating the stability of therapeutic proteins against various interfaces using limited amounts of sample, this method can accelerate the selection of molecules and formulations during early development and formulation design.
[1] Marie Kopp, Umberto Capasso Palmiero, Paolo Arosio, Mol. Pharmaceutics, 2020, 17, 909-918